Deutscher Rheumatologiekongress 2025
Deutscher Rheumatologiekongress 2025
Characteristics, treatment patterns, and discontinuation rates of patients with rheumatoid arthritis treated with baricitinib or other advanced therapies in Germany (RA-BE-REALStudy) at 36-months
Text
Introduction: RA-BE-REAL is a prospective, multinational, observational study, evaluating time until discontinuation following either baricitinib (BARI), biologic disease-modifying antirheumatic drugs (b) DMARDs or other targeted synthetic (ts) DMARDs treatment for the first time. This analysis covers German data.
Methods: Two cohorts were evaluated: treatment with BARI (cohort-A), or any other b/tsDMARDs (cohort-B). Disease activity was assessed by clinical disease activity index (CDAI), physician function by Health Assessment Questionnaire Disability Index (HAQ-DI), and pain by Visual Analogue Scale (VAS). No statistical comparisons were conducted. Time to discontinuation and discontinuation rates were estimated descriptively.
Results: This analysis included 421 (cohort-A; N= 240 and cohort-B; N=181) 3-year data for patients with RA in Germany. In cohort-A, 209 patients received BARI 4mg, and 31 received BARI 2 mg. Cohort-A mean disease duration (standard deviation) of 8.4 (7.3) years was longer than in cohort-B (7.8. [9.4]). Patients previously treated with b/tsDMARDs constituted 48.0% of cohort-A and 32.6% of cohort-B. Monotherapy was more frequent in cohort-A patients (60.8% vs 46.4%) (Table 1 [Tab. 1]). At 36-months, 51.1% (cohort-A) vs 47.2% (cohort-B) of patients achieved low disease activity (CDAI: 2.9-10.0), and 26.7% vs 26.0% achieved remission (CDAI ≤2.8), respectively. Fewer patients in cohort-A (43.1%) discontinued treatment at 36-months vs cohort-B (56.4%). Main reasons for discontinuation were primary non-response (cohort-A; n=13 [5.4%]), cohort-B; n=29 [16.0%]) and secondary loss of response (cohort-A; n=30 [12.6%]) and cohort-B; n=30 [16.6%])* (Table 2 [Tab. 2]).
Table 1: Sociodemographic and clinical characteristics of German patients with RA included in the RA-BE-REAL study.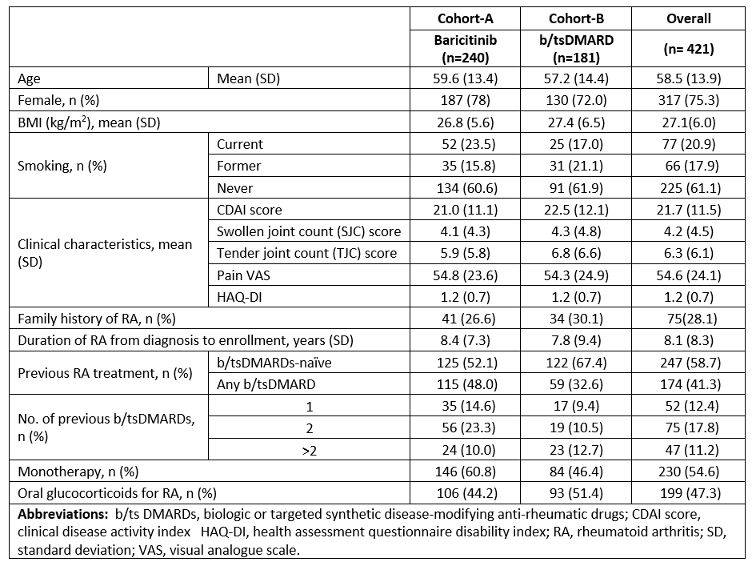
Table 2: Discontinuation rates and reasons for discontinuation of German RA patients included in the RA-BE-REAL study at 36 months*.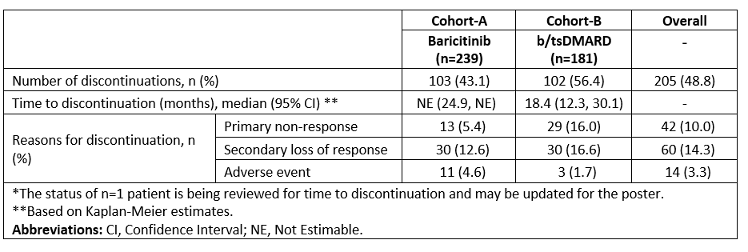
Conclusion: Patients in cohort-A (BARI) had longer disease duration, more frequent use of previous b/tsDMARDs, and more patients in monotherapy. However, fewer discontinuations with less primary and secondary lack of efficacy for up to 3 years were observed in cohort-A. Furthermore, cohort-A showed better effectiveness (CDAI), with a higher percentage of patients achieving low disease activity than cohort-B.
Disclosures: RA reports payment or honoraria for speakers bureaus from: AbbVie, Amgen, BMS, Celltrion, Chugai, Galapagos, Gilead, Janssen, Eli Lilly and Company, Mylan/Viatris, Novartis, Pfizer, Roche, and UCB; consulting fees from: AbbVie, Amgen, BMS, Celltrion, Chugai, Galapagos, Gilead, Janssen, Eli Lilly and Company, Mylan/Viatris, Novartis, Pfizer, Roche, and UCB; and support for attending meetings and/or travel from: AbbVie, Amgen, BMS, Celltrion, Chugai, Galapagos, Gilead, Janssen, Eli Lilly and Company, Mylan/Viatris, Novartis, Pfizer, Roche, and UCB. GB reports speakers bureau: Alfasigma, Abbvie, Galapagos, Eli Lilly and Company, and Pfizer, Consultant of: Abbvie, Galapagos, and Alfasigma. SZ reports fees for studies and lecture from: Abbvie, GSK, Johnson & Johnson, Lilly, and UCB. MS reports COI for Abbvie, AstraZeneca, Böhringer Ingelheimer, BMS, Celltrion, Galapagos, Janssen, Lilly, Novartis, Pfizer, and UCB. KR, AK, JGG, SO, and LS are employees and minor shareholders of Eli Lilly and Company. KK reports payment or honoraria from Lilly and Company as well as consultation fees and clinical trial payments from Eli Lilly and Company.




