Deutscher Rheumatologiekongress 2025
Deutscher Rheumatologiekongress 2025
Development of a physician-independent RA-ILD screening: An oligocentric case-control and monocentric cohort study
Text
Introduction: Interstitial lung disease (ILD) is a leading cause of death in patients with rheumatoid disease (RA). Given the high prevalence of RA, a physician-independent screening is mandatory.
Therefore, this study aimed to provide a patient-based, economical screening questionnaire for rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Methods: A 26-item sum-score questionnaire on ILD-symptoms was evaluated, adapted, and validated in two steps. Multiple imputation handled missing data.
In the first step, an item- and principal component analysis was performed using a multicenter approach in patients with ILD and healthy controls. McDonald’s omega was calculated as a measure of internal consistency. An independent t-test was executed to test for score differences. The effect size was estimated using Cohen’s d.
In the second step, the questionnaire was validated by monocentrically recruited patients with ACPA (anti-citrullinated peptide antibodies) and RF (rheumatoid factor)-positive RA based on ACR criteria, without prior pulmonary examination. ILD was defined as an abnormal lung ultrasound of 14 intercostal spaces with an FVC or DLCO < 80% of the predicted value. Receiver Operating Characteristics (ROC), sensitivity, and specificity were calculated.
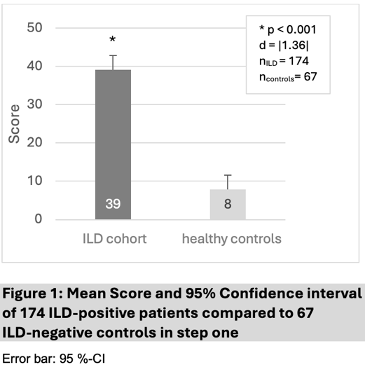
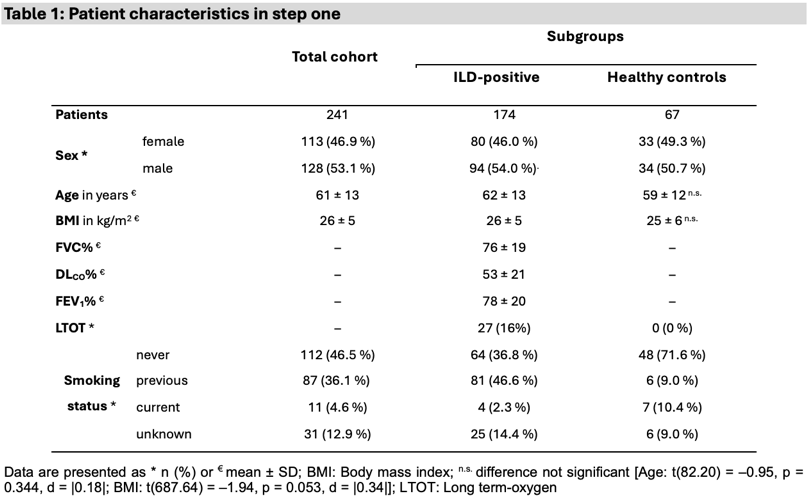
Results: In step one, 174 patients with ILD (46% female, mean age 62 ± 13 years, mean score 39 (95%-CI[35;43]) and 67 healthy controls (49% female, mean age 59 ± 12 years; mean score 8 (95%-CI[4;12]) were recruited. A McDonald's omega of 0.93 indicated excellent internal consistency. The independent t-test revealed a significant score difference of 31 points (95%-CI[26;36]) associated with large effect size, t(85 358.44) = –11.71, p < 0.001, d = |1.36|.
In step two, the questionnaire was validated on 105 patients (76% female, mean age 59 ± 12 years, mean disease duration 9 ± 6 years, mean score 14 (95%-CI[10;17]), 11 of whom (82% female, mean age 65 ± 8 years, mean disease duration 10 ± 5 years, mean score 18 (95%-CI[8;28])) were suspected of having RA-ILD. A cut-off score of 7.5 points resulted in a ROC, sensitivity and specificity of 0.58 (95%-CI[0.39;0.77]), 70.3% (95%-CI[43.5;92.4]), and 47.7% (95%-CI[39.0;59.0]).
Conclusion: The questionnaire showed promising results as a physician-independent, free RA-ILD screening tool for daily clinical practice.
Disclosures: Conflict of interest: RT: Boehringer Ingelheim, FR: Boehringer Ingelheim, CF: Boehringer Ingelheim, M: Boehringer Ingelheim & Roche, JM: None declared, JB: None declared, FP: Boehringer Ingelheim, CD: None declared, SSM: Boehringer Ingelheim, KT: None declared, MW: Boehringer Ingelheim, SW: None declared, NK: Boehringer Ingelheim, MW: Abbvie, BMS, Fresenius, Galapagos, Gilead, GSK, Hexal, Janssen, Johnson & Johnson, Medac, Mylan, Novartis, Sanofi-Aventis, UCB, Viatris, Boehringer Ingelheim, UCD
Figure 1 [Fig. 1]
Table 1 [Tab. 1]