Deutscher Rheumatologiekongress 2025
Deutscher Rheumatologiekongress 2025
Upadacitinib in patients with moderate-to-severe rheumatoid arthritis: 2-year data from Germany, Austria and Switzerland in the observational UPHOLD study
Text
Introduction: Upadacitinib (UPA), an oral reversible JAK inhibitor, has shown efficacy with an acceptable safety profile in patients with RA in the SELECT clinical trials [1]. However, real-world (RW) data on long-term effectiveness and safety of UPA are limited. This sub-analysis of the UPHOLD study aims to evaluate long-term effectiveness and safety of UPA over 2 years in patients with RA in RW practice from Germany, Austria and Switzerland.
Methods: UPHOLD (NCT04497597) is an international, observational cohort study of adults (≥ 18 years old) with moderate-to-severe RA in whom the treating physician decided to initiate treatment with UPA 15 mg once daily prior to study enrolment. Effectiveness endpoints included proportion of patients in each DAS28-CRP/SDAI/CDAI disease category by visit, proportion of patients maintaining DAS28-CRP remission/LDA by treatment strategy and prior therapy exposure, and change from baseline in patient-reported outcomes (PROs). All treatment-emergent adverse events (TEAEs) and selected TEAEs of interest are reported as exposure-adjusted event rates (events per 100 patient-years [E/100PY]).
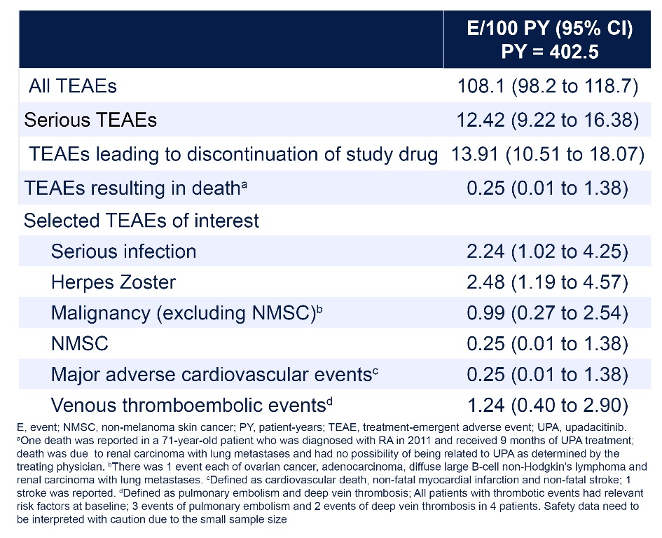
Results: Of the 268 patients enrolled, 262 received ≥ 1 UPA dose and 144 completed the study at 2 years. The most common primary reasons for discontinuation were lack of efficacy (n = 44 [16.4%]) and adverse events (n = 29 [10.8%]). The as-observed proportions of patients achieving DAS28-CRP, SDAI and CDAI remission at 2 years were 68.3%, 38.8% and 37.7%, respectively (Figure 1 [Fig. 1]). Responses were generally similar whether patients were treated with UPA as monotherapy or in combination, and across prior treatment subgroups. Improvements were observed in all assessed PROs at 3 months, which were maintained or further improved through 2 years. A total of 435 TEAEs, including 50 serious TEAEs, were reported by the investigators. Due to small sample size and limited exposure, safety data should be interpreted with caution; however, the safety profile was generally consistent with previous phase 3 long-term clinical trials (Table 1 [Tab. 1]) [2].
Figure 1: Proportions of Patients with RA Achieving Remission Through 24 Months (AO)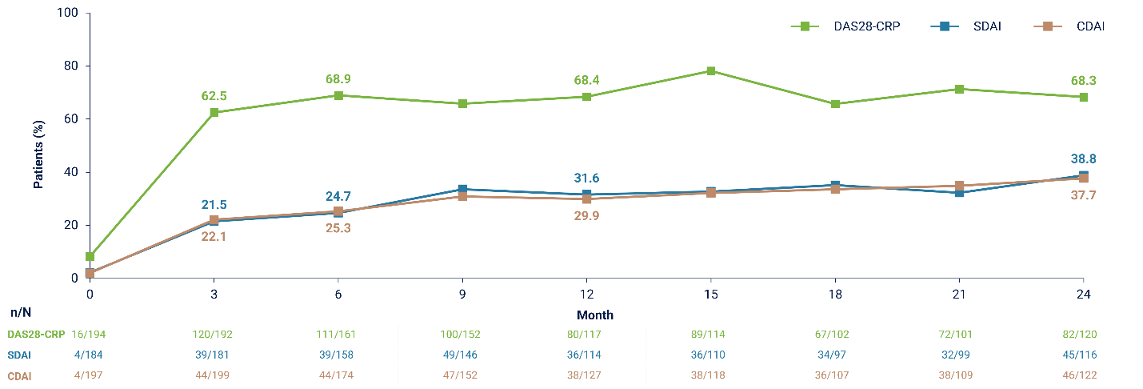
Conclusion: UPA was effective for the treatment of RA in RW practice, of which two thirds achieved sustained DAS28-CRP remission over 2 years. The benefit–risk profile of UPA in this RW setting appears consistent with that demonstrated in phase 3 clinical trials [1], [2].
Literatur
[1] Conaghan PG, Pavelka K, Hsieh SC, Bonnington TL, Kent TC, Marchbank K, Edwards CJ. Evaluating the efficacy of upadacitinib in patients with moderate rheumatoid arthritis: a post-hoc analysis of the SELECT phase 3 trials. Rheumatol Adv Pract. 2023 Feb 8;7(1):rkad017. DOI: 10.1093/rap/rkad017[2] Burmester GR, Cohen SB, Winthrop KL, Nash P, Irvine AD, Deodhar A, Mysler E, Tanaka Y, Liu J, Lacerda AP, Palac H, Shaw T, Mease PJ, Guttman-Yassky E. Safety profile of upadacitinib over 15 000 patient-years across rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis and atopic dermatitis. RMD Open. 2023 Feb;9(1):e002735. DOI: 10.1136/rmdopen-2022-002735