Deutscher Rheumatologiekongress 2025
Deutscher Rheumatologiekongress 2025
Rationale, design and methods of the novel „Filgotinib Initial Response Study in Rheumatoid Arthritis“ (FIRST-RA)
Text
Introduction: FIRST-RA is an observational study designed to evaluate the effectiveness and tolerability of filgotinib in patients with rheumatoid arthritis (RA). While previous randomized controlled trials have established the efficacy of filgotinib [1], [2] real-world data on the early onset of pain relief and functional improvements remain scarce.
The FIRST-RA study therefore aims to assess early pain reduction and patient-reported outcomes with filgotinib therapy in routine clinical practice in Germany and Austria.
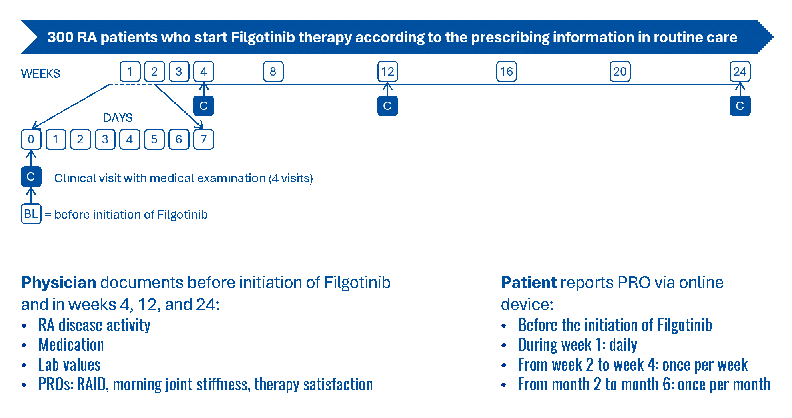
Methods: FIRST-RA [DRKS00036136] is a multicentre, prospective, non-interventional study conducted across approximately 40 rheumatology centres in Germany and Austria.
Eligible patients (n=300) are adults diagnosed with moderate to severe RA for whom filgotinib is newly prescribed as part of their routine care. Patients are enrolled at baseline and followed for 24 weeks to assess treatment response.
To assess early impact of filgotinib treatment, we apply the RAID (Rheumatoid Arthritis Impact of Disease) questionnaire, additional questions for morning stiffness, tolerability and treatment success.
Patients complete the questionnaire using online devices: Before treatment initiation, then daily during the first week, weekly until the end of the first month, and monthly thereafter. Clinical assessments are done at baseline and weeks 4, 12, and 24.
Primary endpoint is the early benefit of filgotinib on pain at week 4 or earlier, compared with baseline. Secondary endpoints include functional improvement, reduced disease activity, fatigue, morning stiffness, treatment satisfaction, and tolerability. Data collection is performed through electronic case report forms (eCRFs) for physicians and patients [3], capturing demographic, clinical, and treatment-related parameters.
Patient characteristics will be compared with those who started filgotinib before the update of the product labelling in March 2023 using a patient cohort from the FILOSOPHY observational study [4].
Results: Pending progress of the study, an interim analysis will provide insights into early treatment response patterns, potential predictors of rapid pain relief and overall filgotinib treatment success.
Conclusion: FIRST-RA aims to generate real-world evidence on rapid symptomatic relief and other improvements provided by filgotinib in RA patients. Findings from this study will support clinicians in optimizing early treatment strategies and personalizing therapeutic approaches to achieve better patient outcomes.
Figure 1 [Fig. 1]
Literatur
[1] Westhovens R. Filgotinib in rheumatoid arthritis. Expert Rev Clin Immunol. 2023 Feb;19(2):135-44. DOI: 10.1080/1744666X.2023.2149495[2] Taylor PC, Kavanaugh A, Nash P, Pope J, Pongratz G, Fautrel B, Alten R, Hasegawa K, Rao S, de Vries D, Stiers PJ, Watson C, Westhovens R. Impact of filgotinib on pain control in the phase 3 FINCH studies. RMD Open. 2024 Mar 12;10(1):e003839. DOI: 10.1136/rmdopen-2023-003839
[3] Krusche M, Klemm P, Grahammer M, Mucke J, Vossen D, Kleyer A, Sewerin P, Knitza J. Acceptance, Usage, and Barriers of Electronic Patient-Reported Outcomes Among German Rheumatologists: Survey Study. JMIR Mhealth Uhealth. 2020 Jul 20;8(7):e18117. DOI: 10.2196/18117
[4] Andreica I, et al. Baseline characteristics, disease activity, patient-reported outcomes and safety in 465 patients with rheumatoid arthritis treated with filgotinib in Germany: Up to 18-month interim results from FILOSOPHY. Presentation at 52nd Congress of DGRh, 18.-21. September 2024, Düsseldorf.