Deutscher Rheumatologiekongress 2025
Deutscher Rheumatologiekongress 2025
Early and sustained improvements in disease activity with filgotinib in patients with rheumatoid arthritis in the real world: 2-year interim effectiveness and safety data from the German FILOSOPHY cohort
Text
Introduction: FILOSOPHY (NCT04871919) is an ongoing, prospective, observational Phase 4 study of filgotinib in Europe. In this interim analysis, data are reported from the German cohort up to 2 years.
Methods: FILOSOPHY enrolled adults with moderate to severe active rheumatoid arthritis who were prescribed filgotinib for the first time in daily practice. Assessments included Clinical Disease Activity Index (CDAI), Disease Activity Score in 28 joints using C-reactive protein (DAS28-CRP), Health Assessment Questionnaire–Disability Index (HAQ-DI) and pain on a visual analog scale (VAS). Reductions of ≥0.22 in HAQ-DI score and ≥10 mm in VAS pain score were considered clinically meaningful changes from baseline. The Kaplan–Meier method was used to estimate treatment persistence up to Month 12. Treatment-emergent adverse events (TEAEs) were reported. All data are on-treatment.
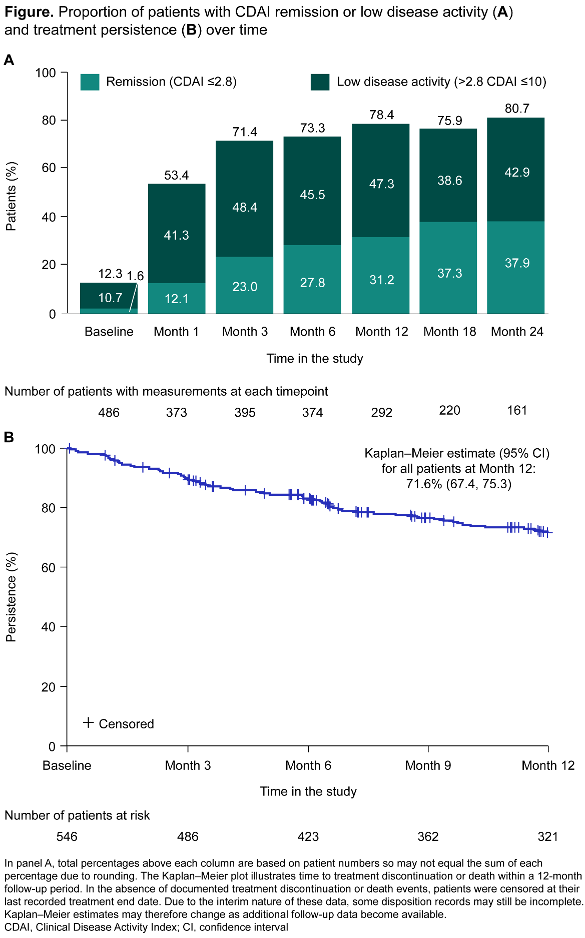
Results: From May 2021 to September 2024, 546 patients were treated with filgotinib (median follow-up: 618 days); baseline characteristics are shown in the Table 1 [Tab. 1]. Remission or low disease activity based on CDAI score (≤10) were reported in 12.3% of patients (60/486) at baseline, increasing to 53.4% (199/373) at Month 1 and 80.7% (130/161) at Month 24 (Figure 1 A [Fig. 1]). DAS28-CRP ≤3.2 was reported in 17.6% of patients (81/461) at baseline, increasing to 59.1% (199/337) at Month 1 and 86.6% (129/149) at Month 24. A clinically meaningful change from baseline in HAQ-DI score was achieved by 28.9% of patients (13/45) at Month 1 and 47.8% (11/23) at Month 12. Corresponding results for VAS pain were 43.5% (80/184) at Week 1 and 64.9% (24/37) at Month 24. Treatment persistence (95% confidence interval [CI]) at Month 12 was 71.6% (67.4, 75.3) (Figure 1 B [Fig. 1]).
Table 1: Baseline characteristics and study treatment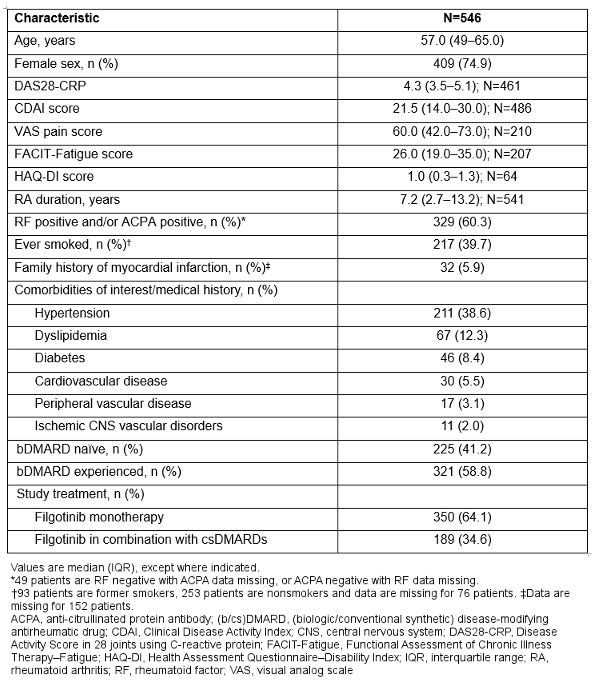
The exposure-adjusted incidence rate (95% CI) per 100 patient-years was 82.9 (74.1, 92.5) for any TEAE and 11.2 (8.7, 14.1) for serious TEAEs.
Conclusion: Patients treated with filgotinib in FILOSOPHY in Germany showed rapid improvements in disease activity, HAQ-DI and VAS pain, which were maintained up to Month 24 (Month 12 for HAQ-DI). Treatment persistence up to Month 12 remained high (71.6%). Safety data were in line with the available evidence, including randomized controlled trials of filgotinib [1], [2], [3].
Acknowledgment: We thank the physicians and patients who participated in the study.
The study was funded by Alfasigma S.p.A. (Bologna, Italy). Medical writing support was provided by Debbie Sherwood, BSc, CMPP (Aspire Scientific, Bollington, UK), and funded by Alfasigma S.p.A.
Literatur
[1] Combe B, Kivitz A, Tanaka Y, van der Heijde D, Simon JA, Baraf HSB, Kumar U, Matzkies F, Bartok B, Ye L, Guo Y, Tasset C, Sundy JS, Jahreis A, Genovese MC, Mozaffarian N, Landewé RBM, Bae SC, Keystone EC, Nash P. Filgotinib versus placebo or adalimumab in patients with rheumatoid arthritis and inadequate response to methotrexate: a phase III randomised clinical trial. Ann Rheum Dis. 2021 Jul;80(7):848-58. DOI: 10.1136/annrheumdis-2020-219214[2] Genovese MC, Kalunian K, Gottenberg JE, Mozaffarian N, Bartok B, Matzkies F, Gao J, Guo Y, Tasset C, Sundy JS, de Vlam K, Walker D, Takeuchi T. Effect of Filgotinib vs Placebo on Clinical Response in Patients With Moderate to Severe Rheumatoid Arthritis Refractory to Disease-Modifying Antirheumatic Drug Therapy: The FINCH 2 Randomized Clinical Trial. JAMA. 2019 Jul 23;322(4):315-25. DOI: 10.1001/jama.2019.9055
[3] Westhovens R, Rigby WFC, van der Heijde D, Ching DWT, Stohl W, Kay J, Chopra A, Bartok B, Matzkies F, Yin Z, Guo Y, Tasset C, Sundy JS, Jahreis A, Mozaffarian N, Messina OD, Landewé RB, Atsumi T, Burmester GR. Filgotinib in combination with methotrexate or as monotherapy versus methotrexate monotherapy in patients with active rheumatoid arthritis and limited or no prior exposure to methotrexate: the phase 3, randomised controlled FINCH 3 trial. Ann Rheum Dis. 2021 Jun;80(6):727-38. DOI: 10.1136/annrheumdis-2020-219213