German Congress of Orthopaedics and Traumatology (DKOU 2025)
Deutscher Kongress für Orthopädie und Unfallchirurgie 2025 (DKOU 2025)
In vitro drug sensitivity testing of bone sarcoma patients
Text
Objectives and questions: Bone-derived sarcomas (BS) are a heterogeneous group of rare malignant tumors that occur throughout the entire skeleton. Current treatment strategies include a variable combination of surgical en bloc resection, (neo)adjuvant chemotherapy (CTx) before and after surgery and radiation therapy. Despite these treatment options, the survival rate of BS patients remains relatively low, underscoring the high medical need for effective treatment strategies. The response to poly-CTx contributes significantly to the treatment success and continues to represent the main predictor for survival. We have developed an ex vivo screening procedure to evaluate the patient-individual response to standard CTx in BS and to cytostatic drugs not commonly used for BS.
Material and methods: A drug sensitivity assay was designed containing 38 drugs or drug combinations in duplicates on 384-well plates. The therapeutic effect of the substances was evaluated in established cell lines before testing patient-derived BS tissue. Primary BS samples were obtained from patients undergoing surgical resection after informed consent. Tumor tissue was processed and seeded at 500–2.000 cells/well. After 96 h, ATP levels were assessed as a surrogate for cell viability.
Results: A drug sensitivity assay was designed and tested in established cell lines. In this pilot experiment, a patient-derived primary BS sample responded to the standard-of-care drug ifosfamide as well as to alvocidib and sirolimus, which are not commonly used in BS treatment (Figure 1 [Abb. 1]). We have collected samples from 35 BS patients including osteosarcoma (n=15), chondrosarcoma (n=18) and chordoma (n=2) to be examined in the drug sensitivity test assay in future experiments. To evaluate the predictability of the data obtained to the actual response in the patient, the results have to be compared with the pathologically identified response of the tumors to the administered cytostatic drugs or drug combinations.
Figure 1: Drug sensitivity test performed on osteosarcoma tissue. The cells responded to alvocidib, ifosfamide and sirolimus.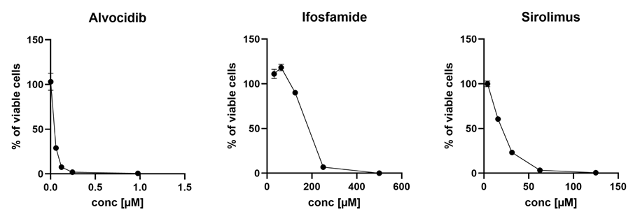
Discussion and conclusions: Our pilot experiments suggest that ex vivo drug sensitivity testing in patient-derived BS models may be a promising approach to predict therapeutic responses. Our approach could pave the way for a potential patient-specific test platform that uses biopsy tissue to provide information on the expected response of the respective patient to certain cytostatic drugs. Pursuing this approach, patients can be stratified to individualized treatment concepts, thereby improving outcome and reducing morbidity. Further studies are warranted to validate these findings and explore the integration of this method into routine clinical practice.




