Deutscher Rheumatologiekongress 2025
Deutscher Rheumatologiekongress 2025
How effective right from the start? A real-world evaluation of the effectiveness of Upadacitinib in patients with axial spondyloarthritis within 12 weeks after treatment initiation
Text
Methods: In this interim analysis alleviation of symptoms was evaluated at weeks 4 and 12 considering the following outcomes: i) ASADAS inactive disease (ID) or low disease activity (LDA) states, ii) ASAS40 response, iii) BASDAI response, and iiii) total back pain. Safety was assessed by collection of adverse event (AE) data.
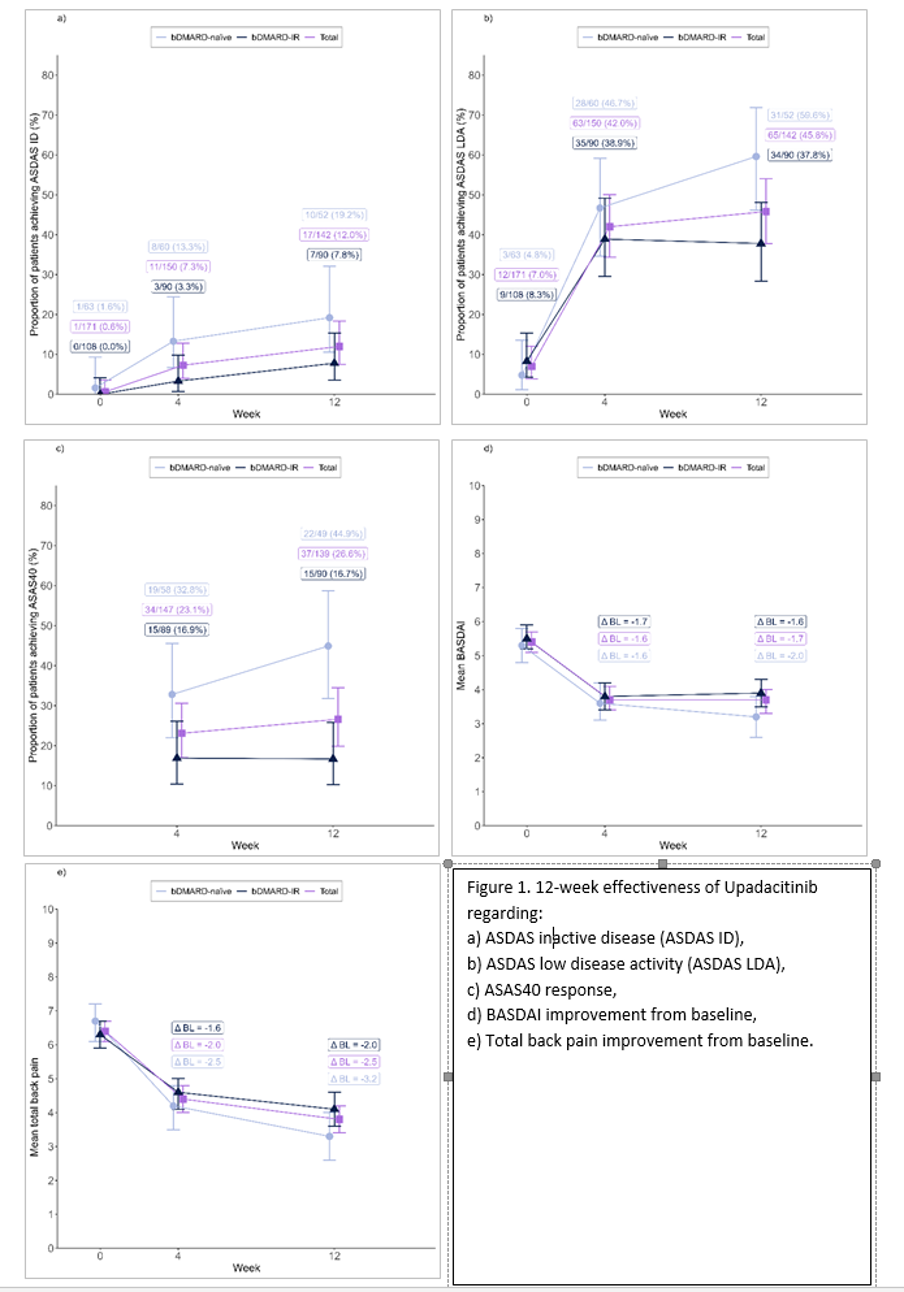
Results: Mean age of 198 patients (59.6% male) was 44.3 (11.8) years, symptom duration was 13.7 (10.5) years and disease duration 9.5 (10.1) years. 39.4% of patients were bDMARD-naïve and 60.6% were bDMARD-IR. Initial ASDAS was 3.4 (0.9) for bDMARD-naïve (n = 63) and 3.2 (0.8) for bDMARD-IRs (n = 108) patients. Baseline CRP-level was 1.2 (2.2) mg/l and decreased to 0.8 (2.0) mg/dl and 0.6 (1.0) mg/dl after 4 and 12 weeks.
ASDAS-ID or ASDAS-LDA increased from 0.6% and 7.0% at baseline to 7.3% and 42.0% at week 4, and to 12.0% and 45.8% at week 12. (Figures 1a & b). 23.1% and 26.6% of all patients achieved ASAS40 at weeks 4 and 12 (Figure 1c). The average BASDAI score of 5.4 (1.9) changed to 3.7 (2.1) at week 4 and week 12, each. (Figure 1d) [1]. Alleviation of total back pain at week 4 was ∆ BL = -2.0 [95%CI: -2.4 to -1.6]) with further pain reduction by week 12 (∆ BL = -2.5 [95%CI: -2.9 to -2.0]) (Figure 1e) [2]. Outcomes for bDMARD-naïve and bDMARD-IR patients were mostly comparable (Figures 1a-e [Fig. 1]). Adverse events did not indicate any new safety signals.
Conclusion: Treatment with UPA led to meaningful improvement of axSpA symptoms demonstrated as early as week 4. Outcomes were comparable for bDMARD-naïve and bDMARD-IR patients. No new safety signals were identified.
Literatur
[1] Kviatkovsky MJ, Ramiro S, Landewé R, Dougados M, Tubach F, Bellamy N, Hochberg M, Ravaud P, Martin-Mola E, Awada H, Bombardier C, Felson D, Hajjaj-Hassouni N, Logeart I, Matucci-Cerinic M, van de Laar M, van der Heijde D. The Minimum Clinically Important Improvement and Patient-acceptable Symptom State in the BASDAI and BASFI for Patients with Ankylosing Spondylitis. J Rheumatol. 2016 Sep;43(9):1680-6. DOI: 10.3899/jrheum.151244[2] Tubach F, Ravaud P, Martin-Mola E, Awada H, Bellamy N, Bombardier C, Felson DT, Hajjaj-Hassouni N, Hochberg M, Logeart I, Matucci-Cerinic M, van de Laar M, van der Heijde D, Dougados M. Minimum clinically important improvement and patient acceptable symptom state in pain and function in rheumatoid arthritis, ankylosing spondylitis, chronic back pain, hand osteoarthritis, and hip and knee osteoarthritis: Results from a prospective multinational study. Arthritis Care Res (Hoboken). 2012 Nov;64(11):1699-707. DOI: 10.1002/acr.21747
[3] van der Heijde D, Deodhar A, Maksymowych WP, Sieper J, Van den Bosch F, Kim TH, Kishimoto M, Östör AJ, Combe B, Sui Y, Duan Y, Wung PK, Song IH. Upadacitinib in active ankylosing spondylitis: results of the 2-year, double-blind, placebo-controlled SELECT-AXIS 1 study and open-label extension. RMD Open. 2022 Jul;8(2):e002280. DOI: 10.1136/rmdopen-2022-002280
[4] Baraliakos X, van der Heijde D, Sieper J, Inman RD, Kameda H, Maksymowych WP, Lagunes-Galindo I, Bu X, Wung P, Kato K, Shmagel A, Deodhar A. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis refractory to biologic therapy: 2-year clinical and radiographic results from the open-label extension of the SELECT-AXIS 2 study. Arthritis Res Ther. 2024 Nov 12;26(1):197. DOI: 10.1186/s13075-024-03412-8
[5] Van den Bosch F, Deodhar A, Poddubnyy D, Maksymowych WP, van der Heijde D, Kim TH, Kishimoto M, Baraliakos X, Li Y, D'Silva K, Wung P, Song IH. Upadacitinib in Active Non-radiographic Axial Spondyloarthritis: 1-Year Data From a Double-Blind, Randomized, Placebo-Controlled, Phase 3 Trial. ACR Open Rheumatol. 2024 Aug;6(8):470-80. DOI: 10.1002/acr2.11669