70. Jahrestagung der Deutschen Gesellschaft für Medizinische Informatik, Biometrie und Epidemiologie e.V.
70. Jahrestagung der Deutschen Gesellschaft für Medizinische Informatik, Biometrie und Epidemiologie e.V.
Instrument linkage in REDCap for study documentation close to routine care within the INTERPOLAR project
2Jena University Hospital, Jena, Germany
3Institute for Medical Informatics, Statistics and Epidemiology (IMISE), Leipzig University, Leipzig, Germany
4Institute for Medical Informatics, Statistics and Epidemiology, Leipzig University, Leipzig, Germany
Text
Introduction: The INTERPOLAR project [1], part of the Medical Informatics Initiative [2], aims to identify medication related problems (MRP) based on documentation from patient care. The EDC-Tool REDCap (Research Electronic Data Capture) [3] acts as a user interface for data documentation and assessment by clinical pharmacists across study sites. Documentation is based on medical information from patient care and assessment relies on processed FHIR resources. We chose REDCap for this purpose due to its high degree of customization, integrability into INTERPOLAR CDS Tool Chain [4] and broad deployment in the international research community. It enables local data collection within the 14 participating centers, while ensuring central data analysis.
Customized data collection instruments (i.e. data entry forms) cover:
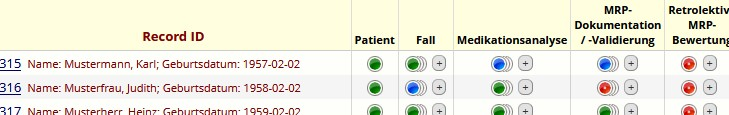
- patient information: “Patient”
- occurring medical cases: “Fall”
- details of performed medication analysis: “Medikationsanalyse”
- resulting pharmaceutical interventions: “MRP-Dokumentation/-Validierung”
- assessment of potential MRP: “Retrolektive MRP-Bewertung”
Example user-overview: Figure 1 [Abb. 1]
A record contains all data collected for a patient and can consist of multiple medical cases, associated medication analyses and MRP. Repeated instances of these instruments can be created for one or multiple cases and their proceedings (overlapping buttons above). To retain the data affiliation between a medical case and its corresponding medication analyses, and the respective MRP detected in each of these analyses, a hierarchical tree-based linkage between these Instruments is required.
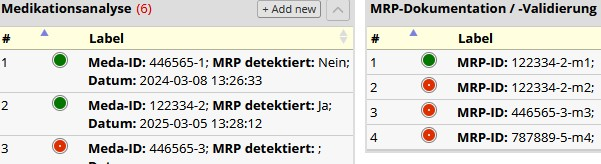
Methods: For instrument linkage, REDCap only offers matching instance numbers (#) of different instruments as a one-to-one relationship, but due to the documentation process, a one-to-many relationship is required, since ‘MRP-Dokumentation’ #1 does not necessarily have to relate to ‘Medikationsanalyse’ #1 (as in the example in Figure 2 [Abb. 2]).
A string function generates a unique ID-chain that is continuously appended to, when automatically transferred to the pending instruments. It is stored in a dropdown menu created by REDCap-SQL-queries.
Results: The ID-chain is based on a unique ID from the hospital information system, which is imported from FHIR-data with all the information for each medical case and transferred to the next related instrument via a dynamic REDCap-function. There it is suffixed with the regarding instance number. ID-transfer refers by default to the latest instance of the preceding instrument. When referencing previous instances, ID-values can be set manually via the SQL-dropdown menus, which display all IDs for an instrument that occur in a record. This method results in a distinct ID-chain for multi-instance linkage between instruments. It provides reliability of automatic transfer and allows manual customization if necessary. Other REDCap with similar data collection structures can also benefit from it.
Conclusion: The lack of connecting IDs was a major issue for data integrity without this implementation. This solution enables traceable data affiliation and provides the basis for distributed multi-site analysis. Difficulties could still arise from the need for user interaction to set IDs manually via SQL-query, but are counteracted by a comprehensive user-manual and the need for a REDCap system admin for its setup and management who might not be affiliated to INTERPOLAR. To overcome remaining challenges, multi-professional communication remains essential for INTERPOLAR and other use cases of the MII.
The authors declare that they have no competing interests.
The authors declare that an ethics committee vote is not required.
Literatur
[1] Loeffler M, Maas R, Neumann D, Scherag A. INTERPOLAR – prospektive, interventionelle Studien im Rahmen der Medizininformatik-Initiative zur Verbesserung der Arzneimitteltherapiesicherheit in der Krankenversorgung. [INTERPOLAR-prospective, interventional studies as part of the Medical Informatics Initiative to improve medication therapy safety in healthcare]. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz. 2024;67:676–84. DOI: 10.1007/s00103-024-03890-w[2] Semler SC, Wissing F, Heyder R. German Medical Informatics Initiative. Methods Inf Med. 2018;57:e50-e56. DOI: 10.3414/ME18-03-0003.
[3] Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap) – a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. DOI: 10.1016/j.jbi.2008.08.010
[4] Stäubert S, Strübing A, Schmidt F, Yahiaoui-Doktor M, Reusche M, Meineke F, et al. The Concept of a Versatile Computing Tool Chain for Utilizing the Core Data Set of the Medical Informatics Initiative in the INTERPOLAR Project. Stud Health Technol Inform. 2024;317:59–66. DOI: 10.3233/SHTI240838