German Congress of Orthopaedics and Traumatology (DKOU 2025)
Deutscher Kongress für Orthopädie und Unfallchirurgie 2025 (DKOU 2025)
Estrogen deficiency exacerbates traumatic heterotopic ossification in mice
Text
Objectives and questions: Traumatic heterotopic ossification (HO) is a devastating sequela following orthopedic surgeries and traumatic injuries, but few studies have explored the effects of the estrogen-deficient state on HO formation. In this study, we investigated the impact of estrogen deficiency on ectopic cartilage and bone formation in tendon after Achilles tenotomy in an ovariectomized mouse model.
Material and methods: A total of 45 female C57BL/6 mice were randomly divided into three groups: sham-operated (control), estrogen depletion by ovariectomy (OVX) and OVX with 17β-estradiol supplementation (OVX + E2), with 15 animals in each group. One week after OVX, all mice were subjected to an Achilles tenotomy using a posterior midpoint approach to induce HO. At 1, 3 and 9 weeks after tenotomy, the left hind limbs were harvested for histology, immunohistochemistry and immunofluorescence evaluations. The volume of ectopic bone was assessed by micro-CT.
Results: Female mice in the OVX group formed more ectopic cartilage at 3 weeks after tenotomy as well as ectopic bone at 9 weeks after tenotomy compared with the control group (Figure 1 [Abb. 1]). The absence of estrogen in vivo resulted in more severe inflammatory infiltration at the injury sites in the early stages of HO, involving the recruitment of more macrophages and mast cells, as well as increasing the expressions of pro-inflammatory mediators including IL-1β, IL-6 and TNF-α. Moreover, local TGF-β/SMAD signaling pathway dysregulated after OVX, which manifested as up-regulated expressions of TGF-β and pSMAD2/3. Furthermore, E2 supplementation could well protect against OVX-induced HO deterioration, inhibit the inflammatory infiltration and downregulate the TGF-β/SMAD signaling pathway at the tenotomy sites.
Figure 1: Estrogen deficiency promoted ectopic bone formation at 9 weeks after tenotomy.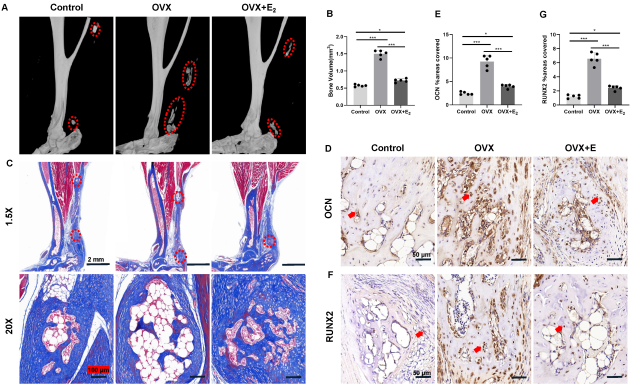
Discussion and conclusions: The estrogen-deficient state achieved by OVX, compared with control mice, led to increased HO formation in the Achilles tenotomy model. These findings may be attributable to the disturbance of the inflammatory response and the activation of TGF-β/SMAD signaling at the injury sites in the early stages of HO development.




